仿生微流體模型——研究血液動力學條件下內皮細胞和血管平滑肌細胞之間的信號傳導
心血管疾病(CVD)是造成死亡最常見的原因之一,因此血管重塑,如動脈硬化中的冠狀動脈重塑顯得非常有必要。血管細胞以及血流力學信號傳導在組織重塑和平衡中起到至關重要的作用。動脈血管壁呈多細胞結構,由內皮細胞(EC)層及其周圍的血管平滑肌細胞(VSMC)組成。目前的3D血管壁構建體不能模擬天然組織的機械條件,也不能在相關的血液動力學條件下監測細胞間相互作用。
基于此,荷蘭埃因霍芬理工大學的Cecilia M. Sahlgren、Nicole C. A. van Engeland團隊建立了動脈內皮細胞和平滑肌細胞的3D微流控芯片模型,其模擬了細胞組成、細胞間相互作用以及動脈壁的機械環境。血液動力學EC-VSMC-芯片上信號傳導由兩個平行的聚二甲基硅氧烷(PDMS)細胞培養通道組成,由柔性多孔PDMS膜隔開,模仿內部彈性薄層的孔隙率(圖 1A-E)。
血流動力學EC-VSMC-芯片上信號傳導允許人主動脈內皮細胞(EC)和人主動脈血管平滑肌細胞(VSMC)的共培養,由多孔膜分離,這使得EC-VSMC相互作用和信號傳導成為可能,這對血管壁的發育和穩態至關重要。該裝置可以實現對細胞實時成像和對血液動力學條件的控制。培養通道兩側均被真空通道包圍(圖 1F),以通過施加循環抽吸誘導循環應變,導致細胞培養通道中膜的機械拉伸和松弛。另外,通過在EC側產生介質流來模擬血流。
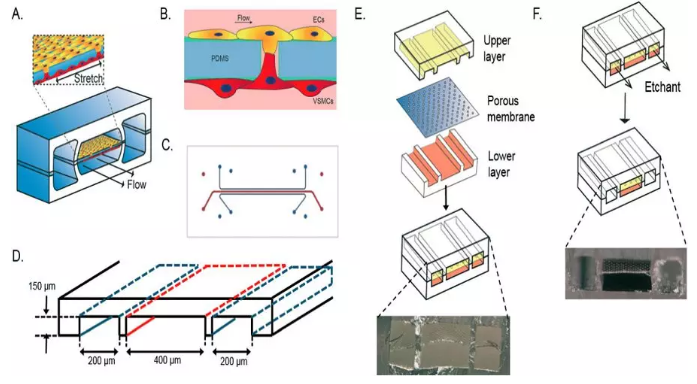
Fig. 1 Schematic overview of the vessel wall on a chip device layer, consisting of two vacuum chambers and a cell culture chamber (A). The microfabricated vessel wall device uses compartmentalized PDMS microchannels to form an organized co-culture of ECs and VSMCs whereby physiological arterial strain and shear stress from the blood flow can be recreated (B). Schematic top view of the final microfluidic device with culture channels (red) and vacuum channels (blue) (C and D). Three PDMS layers are aligned and irreversibly bonded to form two sets of three parallel microchannels, separated by a thin PDMS membrane with pores (E). Selective etching of the membrane layers in the vacuum channels produces two large side chambers to which vacuum is applied, causing mechanical stretching of the membrane in the culture channel (F).
為了確定細胞確實通過基質涂覆的多孔膜接觸,研究人員將EC和VSMC接種在常規裝置和具有完整膜的對照裝置中,并用對其進行免疫熒光染色。通過共聚焦顯微鏡成像顯示EC和VSMC可以通過常規裝置中的膜孔連接,而在對照裝置中細胞不發生相互作用(圖 2)。
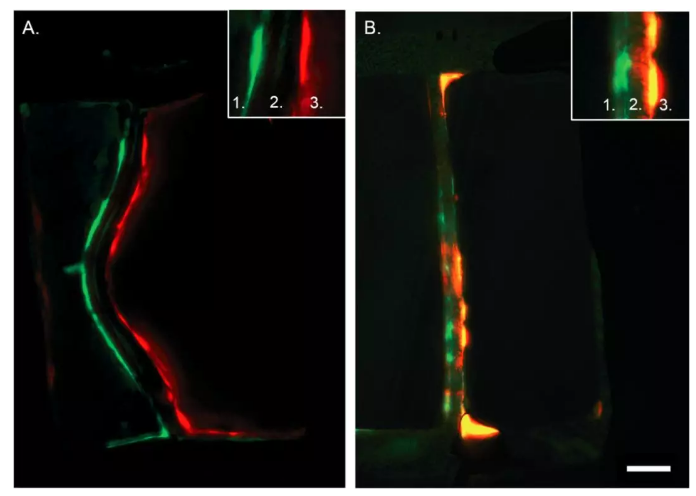
Fig. 2 Cross section of artery-on-a-chip. Immunohistochemistry staining of the device without pores (control, A) and with porous membrane (B). In green the VSMCs and in red the ECs. Number 1 depicts the VSMC side of the device, number 2 the membrane, and number 3 the ECs side. Scalebar represents 50 μm, n = 3–4.
為了研究血液動力學下EC和VSMC的行為,EC和VSMC分別用CellTracker Green和Orange標記,然后種到裝置中。兩種細胞類型在貼附到膜上后表現出隨機排列和類似鵝卵石的形態(圖3 A-D)。當細胞粘附到膜上后,裝置的細胞培養通道連接到壓力驅動的IBIDI系統以在膜的兩側分別保持細胞相應培養基流動。此外,真空通道連接到壓力驅動的IBIDI系統以誘導應變,在脈動血流期間模擬循環周向應變。在動態培養后EC層沒有看到明顯的細胞排列,而VSMC的排列方向與給力方向垂直,且比靜態條件下的細胞更趨于細長的紡錘形(圖3E-H)。
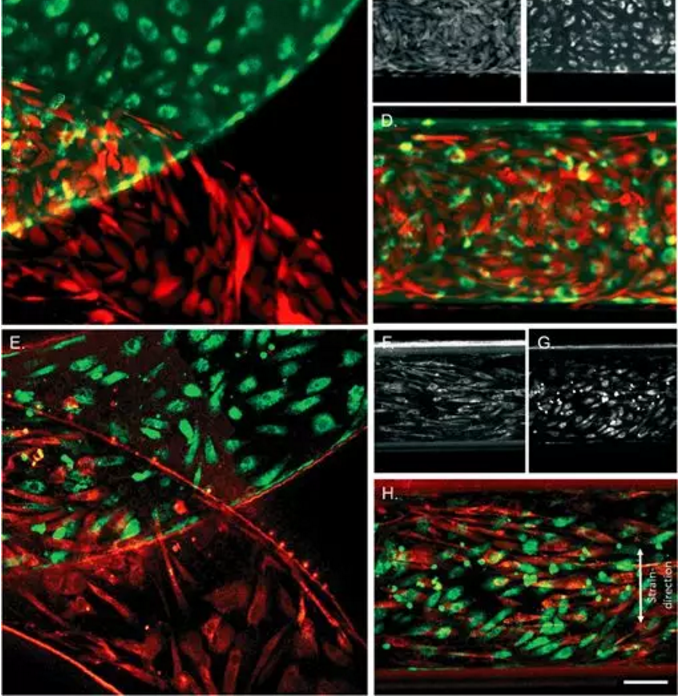
Fig. 3 Live staining of vessel wall on a chip device. A–D. Images were taken directly after adhesion of VSMCs (B) and ECs (C) whereby A and D are merged images. E–H. Live cell stainings after 4 days of dynamic culturing, VSMCs (F), ECs (G) and merged images (E and H). ECs are stained in green and VSMCs in red. Scalebar represents 100 μm, n = 3.
本研究由荷蘭埃因霍芬理工大學的Cecilia M. Sahlgren、Nicole C. A. van Engeland團隊完成,于2018年5月發表于Lab on a Chip。論文鏈接:
http://pubs.rsc.org/-/content/articlelanding/2018/lc/c8lc00286j#!divAbstract(轉載僅供參考學習及傳遞有用信息,版權歸原作者所有,如侵犯權益,請聯系刪除)